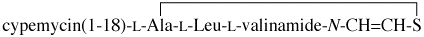
Reaction: cypemycin(1-18)-L-Cys-L-Leu-L-Val-L-Cys + acceptor = C3.19,S21-cyclocypemycin(1-18)-L-Ala-L-Leu-N-thioethenyl-L-valinamide + CO2 + H2S + reduced acceptor
For diagram of reaction click here.
Glossary: C3.19,S21-cyclocypemycin(1-18)-L-Ala-L-Leu-N-thioethenyl-L-valinamide = 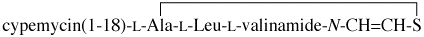
Other name(s): cypemycin decarboxylase; CypD
Systematic name: cypemycin(1-18)-L-Cys-L-Leu-L-Val-L-Cys:acceptor oxidoreductase (decarboxylating)
Comments: Cypemycin, isolated from the bacterium Streptomyces sp. OH-4156, is a peptide antibiotic, member of the linaridins, a class of posttranslationally modified ribosomally synthesized peptides. The enzyme decarboxylates and reduces the C-terminal L-cysteine residue, producing a reactive ethenethiol group that reacts with a dethiolated cysteine upstream to form an aminovinyl-methyl-cysteine loop that is important for the antibiotic activity of the mature peptide.
Links to other databases: BRENDA, EXPASY, KEGG, Metacyc, PDB, CAS registry number:
References:
1. Claesen, J. and Bibb, M. Genome mining and genetic analysis of cypemycin biosynthesis reveal an unusual class of posttranslationally modified peptides. Proc. Natl. Acad. Sci. USA 107 (2010) 16297-16302. [PMID: 20805503]