 . The nature and number of groups covalently linked to the modified enzyme may be indicated by a notation in parenthesis following the name, e.g. (4P) or (12AMP).
. The nature and number of groups covalently linked to the modified enzyme may be indicated by a notation in parenthesis following the name, e.g. (4P) or (12AMP).
World Wide Web version Prepared by G. P. Moss
School of Physical and Chemical Sciences, Queen Mary University of London,
Mile End Road, London, E1 4NS, UK
g.p.moss@qmul.ac.uk
These Rules are as close as possible to the published version [see Arch. Biochem. Biophys., 1978, 185, 1-3; Biochem. J., 1978, 171, 37-39; Eur. J. Biochem., 1978, 82,1-3; J. Biol. Chem., 1977, 252, 5939-5941; and in Biochemical Nomenclature and Related Documents, 2nd edition, Portland Press, 1992, pp 93-95. Copyright IUBMB; reproduced with the permission of IUBMB]. If you need to cite these rules please quote these references as their source.
CBN thanks a subcommittee on Nomenclature of Interconvertible Enzymes whose members were E H Fischer, E. Helmreich, H. G. Hers, B. Hess, H. Holzer (convenor), B. L. Horecker, W. B. Jakoby, E. G. Krebs, J. Larner, L. Reed, A. Sols, E. R. Stadtman, and O. Wieland, for drafting these recommendations.
Any comments should be sent to any member of the Committee
Introduction
I. Definition of Isozymes or Isoenzymes
II. Nomenclature of Isozymes or Isoenzymes
III. Nomenclature of Genetically Variant Enzymes (Allelozymes)
IV. Nomenclature of Interconvertible Enzymes
V. Nomenclature of Enzymes with Proteolytic or other Irreversible Modifications
References
Recommendations on the nomenclature of multiple forms of enzymes were prepared by a subcommittee appointed by the International Union of Biochemistry and published in 1964 in a number of journals (1). These were later revised by a sub-committee of the IUPAC-IUB Commission on Biochemical Nomenclature (CBN) and the revised recommendations were published in 1971 (2).
In view of the rapid development in the field, particularly of conjugated or derived forms of enzymes as related to enzyme regulation, a new subcommittee was appointed to unify the nomenclature of covalently modified enzymes. The report of the subcommittee was received by CBN in 1972 and circulated widely among experts in the field.
The recommendations of the subcommittee, including suggestions received by CBN, were incorporated by CBN into the present set of recommendations covering all multiple forms of enzymes. These recommendations were adopted in 1976.
I. Definition of Isozymes or Isoenzymes
The 1964 Committee recommended (1) that "multiple enzyme forms" in a single species should be known as isoenzymes (or isozymes). It is known that enzymes catalyzing essentially the same reaction may differ in various ways, as shown in Table 1. In this table, some prominent examples are also listed.
According to the original definition, which was meant to be purely operational, all these multiple forms should be termed "isozymes" or "isoenzymes." However, many biochemists feel that the term "isoenzymes" should be restricted to those forms arising from genetic control of primary protein structure. Genetically determined differences in primary structure are the reason for the multiplicity in Groups 1 to 3 of Table 1, but not in Groups 4 to 6. Indeed, investigators working on conjugated or derived enzymes do not use the isozyme terminology for characterization of their multiple forms.
It is therefore recommended that:
1. The term "multiple forms of the enzyme . . ." should be used as a broad term covering all proteins catalyzing the same reaction and occurring naturally in a single species.
2. The term "isoenzyme" or "isozyme" should apply only to those multiple forms of enzymes arising from genetically determined differences in primary structure and not to those derived by modification of the same primary sequence .
II. Nomenclature of Isozymes or Isoenzymes
The 1964 report (1) recommended that individual isoenzymes (isozymes) should be distinguished and numbered on the basis of electrophoretic mobility, with the number 1 being assigned to that form having the highest mobility toward the anode.
Of all the means available to indicate the different properties of isoenzymes (electrophoresis, chromatography, kinetics criteria, chemical structure, etc ), electrophoresis is most widely used for the following reasons.
(a) It is still important in the study of enzyme heterogeneity to avoid any artifactual consequences of the handling or "purification" of enzymes, and zone electrophoresis is one procedure in which the resolution of individual protein species is not unduly influenced by their application in the original state, as tissue homogenates, or the like.
(b) The degree of resolution is another factor of prime importance in this field, and resolution by electrophoretic procedures is generally more effective than other methods of protein separation.
(c) Electrophoresis offers the advantages of rapidity and broad applicability.
The recognition of these facts, and the consequent wide utilization of electrophoretic procedures by workers in this field, has built up a substantial literature on the subject and has facilitated communication and reference.
Other choices suggested for the distinction of isoenzyme forms include kinetic criteria and structural data. While kinetic criteria are valuable as an adjunct to investigations of enzyme multiplicity, they cannot provide information on the extent of heterogeneity. With regard to structural criteria, the ultimate goal is to define the interrelationships of multiple forms of enzymes in chemical terms. However, it is not useful at this stage to provide general recommendations for isoenzyme nomenclature based on structural considerations, because such information is available only for a very few enzyme systems. When structural details become available, notations in upper case Roman letters, as presently used for lactate dehydrogenase and aldolase, may be used. A system similar to that used for hemoglobin, in which the polypeptide chains are represented by Greek letters, would also be acceptable.
Isozymes (isoenzymes) or their subunits should not be labeled on the basis of tissue distribution (e.g. brain type, heart type) since confusion may arise on account of species variation; homologous forms may occur in altogether different tissues in other species.
| Group | Reason of multiplicity | Example |
| 1 | Genetically independent proteinsb | Malate dehydrogenase in mitochondria and cytosol |
| 2 | Heteropolymers (hybrids) of two or more polypeptide drogenase chains, noncovalently boundb | Hybrid forms of lactate dehydrogenases |
| 3 | Genetic variants (allelozymes)b | Glucose-6-phosphate dehydrogenases in man |
| 4 | Conjugated or derived proteins | |
| a. Proteins conjugated with other groups | Phosphorylase b, glycogen synthase a | |
| b. Proteins derived from single polypeptide chains | The family of chymotrypsins arising from chymotrypsinogen | |
| 5 | Polymers of a single subunit | Glutamate dehydrogenase of molecular weight 1,000,000 and 250,000 |
| 6 | Conformationally different forms | All allosteric modifications of enzymes |
b These classes fall into the category of isozymes.
It is therefore recommended that:
3. In naming isozymes (isoenzymes), the normal enzyme name (either systematic or trivial) (3) should be used, followed by a number. The numbers should be allotted consecutively, preferably on the basis of electrophoretic mobility under defined conditions, with the lower numbers given to the forms with the higher mobility towards the anode. In photographs or diagrams of electrophoretic results, the anode and cathode should be clearly identified.
4. Where complex isozyme (isoenzyme) patterns occur, with major groups each composed of several different electrophoretic zones, the numbers may be used to designate the major groups, with subscript lower case letters applied consecutively for the individual subzones (la, lb, 1c, 2a, 2b, etc. ).
5. For unambiguous identification of particular isozymes (isoenzymes), additional characteristics such as molecular weight, stability, or subunit structure should be given where available. Subunits may be denoted by upper case Roman letters or lower case Greek letters, but not by terms based on tissue distribution.
III. Nomenclature of Genetically Variant Enzymes (Allelozymes)
In the case of genetic variants, a flexible system is desirable. The chief consideration is that the investigator recognize that the new variant he describes will probably not be the last one discovered, and in some instances, a very large number may eventually be discovered.
A special committee on the nomenclature of glucose-6-phosphate dehydrogenase in man (of which more than 50 genetic variants are known) was convened by the World Health Organization, and the report of its recommendations was published in abbreviated form in several journals (4); it forms the basis for certain of the recommendations presented here.
This report considers only the naming of the variant enzymes; it does not consider the designation of genotype and phenotype symbols, since these have been standardized by several appropriate groups of geneticists and differ somewhat for specific organisms. The name of the enzyme variant can be readily adapted to the appropriate genetic terminologies.
It is therefore recommended that:
6. In naming genetic variants, the normal enzyme name (either systematic or trivial) should be used, followed by a trivial name for the variant. This can be of any sort provided it is sufficiently adaptable. A suitable system (i.e. that for glucose-6-phosphate dehydrogenase) is to use the name of the town, university, country, etc., in which variant was discovered. The use of superscript letters or numbers is considered acceptable initially, but once it is apparant that more than a few variants are being found, the trivial nomenclature should be introduced, to avoid using the same letter for two different variants .
7. When two variants originally considered as different are subsequently discovered to be identical, the name first used for that variant should be accepted and the second discontinued .
IV. Nomenclature of Interconvertible Enzymes
The following definitions of interconversion and of converting enzymes are recommended:
8. An "interconvertible enzyme" is one existing in at least two well defined, reversibly convertible forms, produced by covalent modifications of amino acid side chains under biological conditions. Covalent modifications that occur as intermediates in the catalytic process are not included in this definition.
9. Evidence should be presented for "interconversion" if this is considered to be brought about by enzymes, although such enzyme catalysis might not be required in the case of forms differing by the presence or absence of -SS- bridges.
For the designation of interconvertible forms, the following conventions are recommended:
10 Interconvertible forms may be designated by o for the unmodified (original, containing unmodified amino acid residues) and m for modified forms, preceding the name of the enzyme. For interconvertible enzymes with disulfide linkages, the -SH form is designated as o and the -SS- form as m.
11. Oligomeric enzymes involving two or more modifiable subunits (hybrid forms) may be described by the appropriate letters o and m followed by a numerical subscript indicating the number of subunits in each form, e.g. on, on-1m, on-2m2 . . . o2mn-2, omn-1, mn.
12. If the composition of a hybrid is not known to represent a defined subunit stoichiometry, but is an average of a mixture of oligomers of different subunit stoichiometry, this may be indicated by use of bars placed over the letters. For example, 10% adenylylated glutamine synthetase, which is composed of 12 subunits, is written  . The nature and number of groups covalently linked to the modified enzyme may be indicated by a notation in parenthesis following the name, e.g. (4P) or (12AMP).
. The nature and number of groups covalently linked to the modified enzyme may be indicated by a notation in parenthesis following the name, e.g. (4P) or (12AMP).
13. If desired, changes in activity may be indicated by the letters a for active and b for inactive or less active, following the name of the enzyme.
| a. For glutamine synthetase | |
| Unmodified form (o = original) | Modified form (m = modified) |
| o (or o12)-glutamine synthetase or, o-glutamine synthetase a | m (or m12)-glutamine synthetase, m-glutamine synthetase b, or m-glutamine synthetase(l2AMP) |
| Intermediate forms | |
| o6m6-glutamine synthetase (defined composition) | |
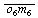 -glutamine synthetase(6AMP) (undefined composition) -glutamine synthetase(6AMP) (undefined composition) | |
| b. For phosphorylase | |
| Unmodified form (original) | Modified form (modified) |
| o-phosphorylase, o-phosphorylase b, or o2-phosphorylase (dimer) | m-phosphorylase, m-phosphorylase a, or m-phosphorylase(4P) |
| o4-phosphorylase (tetramer) | m2-phosphorylase (dimer) m4-phosphorylase (tetramer) |
| Hybrids | |
| om-phosphorylase (dimer) | |
| o2m2-phnsphorylase (tetramer) | |
 inhibition and
inhibition and  stimulation
stimulation
 repression and
repression and  induction
induction
(o m), interconvertible enzyme
V. Nomenclature of Enzymes with Proteolytic or other Irreversible Modifications
Multiple forms of enzymes occur as the result of proteolytic or other irreversible modification under biological conditions.
For those enzymes in which an inactive precursor is converted to the active form(s), it is recommended that:
15. The use of the term zymogen and the suffix -ogen be retained for the enzyme precursor or pro-enzyme (e.g. trypsinogen, trypsin, chymotrypsinogen, chymotrypsin). When the modification leads to a family of enzymes, these may be designated by the prefix m carrying the Greek letter subscript, to avoid confusion with number of subunits (e.g. m-chymotrypsin, m
-chymotrypsin, etc.).
16. When the original protein and the modified form(s) both possess catalytic activity, the o and m designation should be used, as recommended for the interconvertible forms.
1. Webb, E. C. (1964) Lancet 1, 1110; (1964) Nature (Lond.) 203, 821; (1964) Experientia 20, 592; (1964) Z. Klin. Chem. 2, 160; (1964) Postepy Biochem. 10, 525; (1965) Enzymol. Biol. Clin. 5, 124
2. The Nomenclature of Multiple Forms of Enzymes; Recommendations (197l), based on the report of a subcommittee consisting of G. Brewer, O. Hoffmann-Ostenhof, R. S. Holmes, P. Karlson (Convenor), B. Keil, G. B. Kitto, C. L. Markert, C. J. Masters, F. Moyer, J. Scandaliofi, C. R. Shaw, E. C. Slater, R. Tashian, and E. C. Webb. The document was published in the following journals: (1971) Arch. Biochem. Biophys. 147, 1; (1971) Biochem. J. 126, 769; (1971) Biochemistry 10, 4825; (1971) Biochimie 54, 123; (1971) Biochim. Biophys. Acta 258,1; (1971) Eur. J. Biochem. 24, 1; (1971) Hoppe-Seyler's Z. Physiol. Chem. 353, 852; (1971) J. Biol. Chem. 246, 6127; (1974) Pure Appl. Chem. 40, 309 (1974)
3. Enzyme Nomenclature: Recommendations (1972) of the International Union of Pure and Applied Chemistry and the International Union of Biochemistry on the nomenclature and classification of enzymes, together with their units and the symbols of enzyme kinetics (1973), Elsevier Publishing Co., Amsterdam
4. (1967) Biochem. Genet. 1, 198