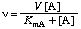 . . . . . . . . (10)
. . . . . . . . (10)
Contents of Section
4.1. Limiting Kinetics of Enzyme-Catalysed Reactions
At very low concentrations of substrate many enzyme-catalysed reactions display approximately second-order kinetics, with rate given by the following equation:
v = kA [E]0 [A] . . . . . . . . (8)
in which the symbol kA (or, in general, kR for a reactant R) is the apparent second-order rate constant or specificity constant and [E]0, which may also be written as [E]t or [E]stoich, is the total or stoichiometric concentration of catalytic centres. (This corresponds to the total enzyme concentration only if there is a single catalytic centre per molecule.) The rationale for the subscript 0 is that the total enzyme concentration is normally the concentration at the instant of mixing, i.e. at time zero. Conversely, at very high substrate concentrations the same reactions commonly display approximately first-order kinetics (zero-order with respect to substrate):
v = k0 [E]0 . . . . . . . . (9)
in which k0, which may also be written as kcat is the apparent first-order rate constant. Although these limiting types of behaviour are not universally observed, they are more common than Michaelis-Menten kinetics (Section 4.2) and provide a basis for classifying inhibitory and other effects (Section 5) independently of the need for Michaelis-Menten kinetics.
The apparent second-order rate constants kA and kB of competing substrates A and B determine the partitioning between competing reactions, regardless of whether the substrate concentrations are very small or not, and it is for this reason that the name specificity constant is proposed for this parameter of enzymic catalysis. The apparent first-order rate constant k0 is a measure of the catalytic potential of the enzyme and is called the catalytic constant.
The quantity k0[E]0 is given the symbol V and the name limiting rate. It is particularly useful when k0 cannot be calculated because the total catalytic-centre concentration is unknown, as in studies of enzymes of unknown purity, sub-unit structure and molecular mass. The symbol Vmax and the names maximum rate and maximum velocity are also in widespread use although under normal circumstances there is no finite substrate concentration at which v = V and hence no maximum in the mathematical sense. The form Vmax is convenient in speech as it avoids the need for a cumbersome distinction between 'capital V' and 'lower case v'. When a true maximum does occur (as insubstrate inhibition; Section 4.3) the symbol vmax (not Vmax) and the name maximum rate may be used for the true maximum value of v but care should be taken to avoid confusion with the limiting rate.
4.2. Michaelis-Menten Kinetics
Sometimes the relationship between the rate of an enzyme-catalysed reaction and the substrate concentration takes the form
. . . . . . . . (10)
where V and KmA are constants at a given temperature and a given enzyme concentration. The reaction is then said to display Michaelis-Menten kinetics. (The term hyperbolic kinetics is also sometimes used because a plot of v against [A] has the form ot a rectangular hyperbola through the origin with asymptotes v = V and [A] = -KmA. This term, and others that imply the use of particular kinds of plot. should be used with care to avoid ambiguity, as they can be misleading if used out of context.) The constant V is the limiting rate, with the same meaning as in Section 4.1. The second constant KmA is known as the Michaelis constant for A; the alternative name Michaelis concentration may also be used and has the advantage of emphasizing that the quantity concerned has the dimensions of a concentration and is not, in general, an equilibrium constant. When only one substrate is being considered the qualifier A may be omitted, so that the symbol becomes Km. When the qualifier is included its location is a matter of typographical convenience; no particular significance attaches to such variants as 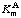 or KmA. The Michaelis constant (or Michaelis concentration) is the substrate concentration at which v = 0.5 V, and its usual unit is mol dm-3, which may be written as mol L-1 or M. The term Michaelis constant and the symbol Km should not be used when Michaelis-Menten kinetics are not obeyed (see Section 4.3).
or KmA. The Michaelis constant (or Michaelis concentration) is the substrate concentration at which v = 0.5 V, and its usual unit is mol dm-3, which may be written as mol L-1 or M. The term Michaelis constant and the symbol Km should not be used when Michaelis-Menten kinetics are not obeyed (see Section 4.3).
For a reaction obeying Michaelis-Menten kinetics the rate in the limit of very low substrate concentrations is v = V[A]/KmA, and comparison with Eqn (8) shows that V/KmA = kA [E]0. In the limit of very high substrate concentrations v = V, and comparison with Eqn (9) gives V = k0 [E]0. The Michaelis constant KmA is therefore k0/kA, and Eqn (10) can be written as
. . . . . . . . (11)
An indefinitely large number of mechanisms generate Michaelis-Menten kinetics, and still more generate limiting behaviour of the kind described in Section 4.1. Consequently there is no general definition of any of the kinetic parameters kA, k0, V and KmA in terms of the rate constants for the elementary steps of a particular mechanism.
4.3. Non-Michaelis-Menten Kinetics
When the kinetic behaviour does not conform to Eqn (10) or Eqn (11) the reaction is said to exhibit non-Michaelis-Menten kinetics. If the Michaelis-Menten equation is obeyed approximately over a restricted range of substrate concentrations it may be convenient to regard this behaviour as a deviation from this equation rather than as an unrelated phenomenon. For example, a reaction may obey an equation of the following form
. . . . . . . . (12)
in which the constants V', K'mA and KiA are used for illustration without any implication of universal or standard definitions. If KiA is large compared with KmA the behaviour predicted by Eqn (12) will approximate to that predicted by Eqn (11), with V and KmA replaced by V' and KmA, in the lower range of substrate concentrations. However, with Eqn (12) the rate passes through a maximum as the concentration increases, and there is said to be inhibition by substrate, and the constant KiA, which has the dimensions of a concentration, is called the substrate inhibition constant.
When more complex kinds of non-Michaelis-Menten behaviour occur it is usually unhelpful to use terminology and symbolism suggestive of the Michaelis-Menten equation; instead the approach discussed in Section 10 is appropriate. In all cases it is advisable to avoid the term Michaelis constant and the symbol Km when the Michaelis-Menten equation is not obeyed, because it is defined as a parameter of that equation. The symbol [A]0.5 or [A]1/2, not KmA, may be used for the value of [A] at which v = 0.5 V.
5. ENZYME REACTIONS INVOLVING MORE THAN ONE SUBSTRATE
5.1. Michaelis-Menten Kinetics
Regardless of the number of substrates, a reaction is said to obey Michaelis-Menten kinetics if the rate equation can be expressed in the following form:
. . . . . . . . (13)
which can be regarded as a generalization of Eqn (11). (Z is used here as an example of a product as suggested in Section 2.) Each term in the denominator of the rate expression contains unity or any number of product concentrations in its numerator. and a coefficient k and any number of substrate concentrations raised to no higher than the first power in its denominator. The constant k0 corresponds to k0 in Eqn (11); each other coefficient is assigned a subscript for each substrate concentration in the denominator of the term concerned and a superscript for each product concentration in the numerator. The term 1/k0 must be present, together with one term for each substrate of the form 1/kA [A], but the terms in products of concentrations. such as those shown in Eqn ( 13) with coefficients kAB and 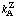 , may or may not be present. It is sometimes convenient to write the equation in a form in which each k is replaced by its reciprocal, symbolized by
, may or may not be present. It is sometimes convenient to write the equation in a form in which each k is replaced by its reciprocal, symbolized by with the same subscripts and superscripts, i.e.
![[phi 0]](ekgif/phi0.gif) = l/k0,
= l/k0, ![[phi A]](ekgif/phia.gif) = l/kA,
= l/kA, ![[phi AB]](ekgif/phiab.gif) = 1/kAB,
= 1/kAB, 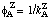 , etc. These reciprocal coefficients are called Dalziel coefficients.
, etc. These reciprocal coefficients are called Dalziel coefficients.
Note The conventional Scottish pronunciation of this name may be expressed in the International Phonetic Alphabet as [di:'jel], with only slightly more stress on the second syllable than the first.
Eqn (13) can be applied to reactions with any number of substrates and products and can also be extended to some kinds of inhibition by substrate, i.e. to the simpler kinds of non-Michaelis-Menten kinetics. It is thus an equation of considerable generality. It is simplest, however, to consider terminology in the context of a two-substrate reaction, and this will be done in Section 5.2.
5.2. Michaelis-Menten Kinetics of a Two-Substrate Reaction
For a two-substrate reaction in the absence of products Eqn (13) simplifies to the following equation:
. . . . . . . . (14)
If the concentration of one substrate, known as the constant substrate, is held constant, while that of the other, known as the variable substrate, is varied, the rate is of the form of the Michaelis-Menten equation in terms of the variable substrate, because Eqn (14) can be rearranged to
. . . . . . . . (15)
(cf. Eqn 10), where
. . . . . . . . (16)
is known as the apparent catalytic constant, and
. . . . . . . . (17)
is known as the apparent specificity constant for A. It follows from Eqns (16) and (17) that 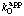 = k0 and
= k0 and 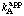 = kA when [B] is extrapolated to an infinite value. This relationship provides the basis for defining the catalytic constant and the specificity constants in reactions with more than one substrate: in general, the catalytic constant of an enzyme is the value of v/[E]0 obtained by extrapolating all substrate concentrations to infinity; for any substrate A the specificity constant is the apparent value when all other substrate concentrations are extrapolated to infinity.
= kA when [B] is extrapolated to an infinite value. This relationship provides the basis for defining the catalytic constant and the specificity constants in reactions with more than one substrate: in general, the catalytic constant of an enzyme is the value of v/[E]0 obtained by extrapolating all substrate concentrations to infinity; for any substrate A the specificity constant is the apparent value when all other substrate concentrations are extrapolated to infinity.
Eqn (14) may also be rearranged into a form resembling Eqn (11), as follows:
. . . . . . . . (18)
in which V = k0[E]0 is the limiting rate, which may also, subject to the reservations noted in section 4.1, be called the maximum rate or maximum velocity and symbolized as Vmax; KmA = k0/kA is the Michaelis constant for A; KmB = k0/kB is the Michaelis constant for B; and KiA = kB/kAB is the inhihition constant for A. In some mechanisms KiA is equal to the true dissociation constant for the EA complex: when this is the case the alternative symbol KiA and the name substrate-dissociation constant for A (cf. section 3.2) may be used. If Eqn(18) is interpreted operationally rather than as the equation for a particular mechanism it is arbitrary whether the constant in the denominator is written with KiAKmB (as shown) or as KmAKiB, where KiB = kA/kAB. However, for some mechanisms only one of the two ratios kB/kAB and kA/kAB has a simple mechanistic interpretation and this may dictate which inhibition constant it is appropriate to define.
The term inhibition constant and the symbol KiA derive from the fact that the quantity concerned is related to (and in the limiting cases equal to) the inhibition constant Kic or Kiu (as defined below in Section 6.4) measured in experiments where the substrate is treated as an inhibitor of the reverse reaction. However, the relationships are not always simple and quantities such as KiA in Eqn (18) can be and nearly always are defined and measured without any reference to inhibition experiments. For these reasons some members of the panel feel that the symbolism and terminology suggested are not completely satisfactory. No alternative system has so far gained wide support, however.
An apparent Michaelis constant for A (and similarly for B) may be defined by dividing Eqn (16) by Eqn (17):
. . . . . . . . (19)
This equation provides the basis for defining the Michaelis constant for any substrate in a reaction with more than one substrate: the Michaelis constant for A, KmA, is the value of the apparent Michaelis constant for A when the concentrations of all substrates except A are extrapolated to infinity. This definition applies to reactions with any numbers of substrates, as also does the definition of the limiting rate V as k0 [E]0, but in other respects it becomes very cumbersome to define constants resembling KiA for reactions with more than two substrates. The symbolism of Eqn (13) (or the alternative in terms of Dalziel coefficients) is readily extended to reactions with three or more substrates, however.
6.1. Reversible and Irreversible Inhibitions
Sometimes the effect of an inhibitor can be reversed by decreasing the concentration of inhibitor (e.g. by dilution or dialysis). The inhibition is then said to be reversible. If, once inhibition has occurred, there is no reversal of inhibition on decreasing the inhibitor concentration the inhibition is said to be irreversible; irreversible inhibition is an example of enzyme inactivation. The distinction between reversible and irreversible inhibition is not absolute and may be difficult to make if the inhibitor binds very tightly to the enzyme and is released very slowly. Reversible inhibitors that behave in a way that is difficult to distinguish from irreversible inhibition are called tight-binding inhibitors.
6.2. Linear and Non-Linear Inhibition
Sometimes the effect of an inhibitor I can be expressed by multiplying one or more of the terms in the denominator of the general rate expression (Eqn 13) by factors of the form (1 + [I]/Ki). The inhibition is then said to be linear and Ki which has the dimensions of a concentration, is called an inhibition constant for the inhibitor I. The word linear in this definition refers to the fact that the inhibition is fully specified by terms in the denominator of the rate expression that are linear in inhibitor concentration, not to the straightness of any plots that may be used to characterize the inhibition experimentally.
If the inhibition cannot be fully expressed by means of linear factors in the denominator the inhibition is said to be non-linear.
Linear inhibition is sometimes called complete inhibition, and the contrasting term partial inhibition is sometimes used for a type of non-linear inhibition in which saturation with inhibitor does not decrease the rate to zero. These latter terms are discouraged because they can be misleading, implying, for example, that the rate may indeed be decreased to zero in 'complete inhibition' at non-saturating concentrations of inhibitor.
If a reaction occurs in the absence of inhibitor with rate v0 and in the presence of inhibitor with rate vi, the degree of inhibition is defined as
. . . . . . . . (20)
As this quantity is a ratio of rates it is dimensionless. The subscripts '0' and 'i' are useful for distinguishing between uninhibited and inhibited reactions respectively when they are required together, but are usually omitted when no confusion is likely.
6.4. Classification of Inhibition Types
Provided that an enzyme behaves in accordance with the limiting behaviour described in Section 4.1 both in the absence of inhibitor (which is always true if Michaelis-Menten kinetics are obeyed and is also true more generally), the type of inhibition may be classified according to whether it affects the apparent value of kA, the apparent value of k0, or both.
If the apparent value of kA is decreased by the inhibitor the inhibition is said to have a competitive component, and if the inhibitor has no effect on the apparent value of k0 the inhibition is said to be competitive.. In linear inhibition there is a linear effect on 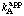
. . . . . . . . (21)
and the constant Kic is called the competitive inhibition constant for I.
Conversely, if there is an effect on the apparent value of k0 the inhibition has an uncompetitive component, and if the innibitor has no effect on the apparent value of kA the inhibition is said to be uncompetitive. In linear inhibition there is a linear effect on 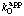
. . . . . . . . (22)
and constant Kiu is called uncompetitive inhibition constant for 1.
If both competitive and uncompetitive components are present in the inhibition it is said to be mixed. The term non-competitive inhibition is sometimes used instead of mixed inhibition, but this usage is discouraged, first because the same term is often used for the special case of mixed inhibition in which Kic = Kiu, second because it suggests that mixed inhibition is the antithesis of competitive inhibition whereas this description actually applies more accurately to uncompetitive inhibition, and third because the shorter word mixed expresses clearly the fact that both competitive and uncompetitive components are present.
Mixed inhibition as defined here encompasses such a broad range of behaviour that it may sometimes be helpful to subdivide it further. The case in which Kic < Kiu may then be called predominantly competitive inhibition, the case with Kic = Kiu may be called pure non-competitive inhibition, and the case with Kic > Kiu may be called predominantly uncompetitive inhibition. The classical term for pure non-competitive inhibition was simply non-competitive inhibition, but this term has become ambiguous because of its widespread use for all kinds of mixed inhibition and because of this ambiguity it is discouraged for all purposes.
Both Kic and Kiu have the dimensions of concentrations and may therefore be expressed in mol dm-3, mol L-1 or M. In contexts where distinction between Kic and Kiu is unnecessary or inappropriate the general symbol Ki may be used for either. In the past there has been no generally understood symbol for the uncompetitive inhibition constant, which has been variously represented as Ki, K'i, Kii, etc. A new and unambiguous symbol seems required, therefore, and Kiu is proposed. Although the competitive inhibition constant has much more uniformly been expressed as Ki, the occasional use of the same symbol for the uncompetitive inhibition constant, together with the view that a logical and symmetrical symbolism is desirable, has suggested that the symbol Kic should be used for the competitive inhibition constant whenever any ambiguity might attend the use of the more general symbol Ki.
As Kic and Kiu can in principle be determined by measuring the effects of inhibitor on the slopes and ordinate intercepts respectively of plots of 1/v against 1/[A] they, have sometimes been symbolized as Kis (for Ki slope) and Kii (for Ki intercept) respectively. Slopes and intercepts are not consistent from one kind of plot to another, however; for example, the slope and intercept in a plot of [A]/v against [A] correspond, respectively, to the intercept and slope of a plot of 1/v against 1/[A]. Such symbols are therefore ambiguous and should not be used except in explicit reference to particular plots.
In reactions with more than one substrate the classification of inhibitors as competitive, uncompetitive or mixed is not absolute but depends on which substrate is variable (in the sense of Section 5.2). For example, a particular inhibitor may cause variation in 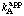 without any variation in
without any variation in 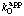 when A is the variable substrate, but cause variation in both
when A is the variable substrate, but cause variation in both 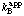 and
and 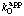 when B is the variable substrate: it is then said to be a competitive inhibitor with respect to A but a mixed inhibitor with respect to B. In such systems the inhibition constants Kic and Kiu refer to the limiting behaviour for saturating concentrations of all substrates except for the variable substrate. Inhibition constants observed at non-saturating concentrations of the constant substrates are apparent values and may be symbolized as
when B is the variable substrate: it is then said to be a competitive inhibitor with respect to A but a mixed inhibitor with respect to B. In such systems the inhibition constants Kic and Kiu refer to the limiting behaviour for saturating concentrations of all substrates except for the variable substrate. Inhibition constants observed at non-saturating concentrations of the constant substrates are apparent values and may be symbolized as 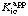 and
and 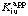 .
.
For some mechanisms some inhibition constants may be true dissociation constants. Whether this is true or not it does not form part of the definitions of the inhibition types and inhibition constants given above, which are purely operational, in keeping with the policy set out in Section 1. When symbols are required for the dissociation constants of particular species they should be explicitly defined in a way that avoids confusion with the operationally defined inhibition constants. A system of the following kind may be appropriate, but if used it should be explicitly defined in context. For a binary complex, e.g. EI, the dissociation constant may be symbolized as K with the name of the complex as subscript, e.g. KEI For higher complexes where the nature of the dissociation needs to be specified, a full stop (period) may be used to separate the parts of the complex that dissociate from one another; for example, KEI.S may be used for the dissociation of EIS into EI + S, whereas KES.I may be used for the dissociation of the same complex into ES + I.
The products of nearly all enzyme-catalyzed reactions behave as inhibitors when they are present in the reaction mixture. When considered in this light they are called product inhibitors and the phenomenon is known as product inhibition. Product inhibition is always reversible (at least in principle) but in other respects occurs in the same varieties as other kinds of inhibition and requires no special discussion or definitions.
Return to home page for Enzyme Kinetics.